Messenger RNA (mRNA)-based biotechnologies have seen rapid recognition and expansion since the COVID-19 pandemic and the successful launch of the first mRNA vaccines. The unique advantages of mRNA drugs for protein expression, resulting from decades of research and development, are now promising exciting opportunities and rapid progress in many areas of medicine, from vaccines to therapeutics.
The single and swift process for mRNA synthesis – centered on cell-free in vitro transcription (IVT) from a precision DNA template – enables rapid and efficient access to a wide variety of mRNA structures potentially capable to address a large number of (so far intractable) diseases, thereby opening a new era in modern medicine. Following proper design, synthesis, purification and formulation, synthetic mRNA can be effectively transfected and translated into functional proteins using the patient own cellular machinery, alleviating the need to synthesize and deliver therapeutic peptides and proteins instead.
However, to realize the full potential of mRNA-based medicines, different challenges still need to be addressed – from optimization of the mRNA sequence, IVT synthesis and final encapsulation – in order to increase stability, enhance protein expression level and achieve targeted delivery when needed. In parallel, it becomes critical to devise innovative and robust manufacturing approaches to accelerate the development and production of mRNA at scale.
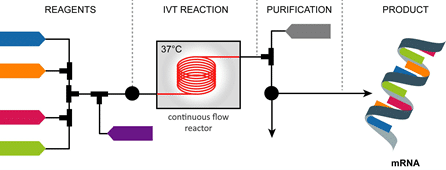
In this direction, in a recent collaboration with Dillico, Certech is exploring the implementation of process intensification and continuous flow technologies – some of Certech’s core expertise – for transforming mRNA manufacturing. In this new paradigm, the different steps of mRNA production (synthesis, purification and encapsulation), conventionally performed sequentially in batch reactors, will be combined in a single integrated flow for enhanced process efficiency, control, quality, flexibility and scalability. The resulting miniaturized, automated and digitalized continuous flow platform will allow on-site on-demand mRNA production at different scales, from clinical to commercial.
Especially, Dillico and Certech have focused on the development of a custom microfluidic reactor module dedicated to the IVT reaction, central to the production of mRNA. Preliminary results confirmed the synthesis of mRNA in continuous flow; further efforts are underway to optimize the design and operation of the flow process.